We recently put this to the test.
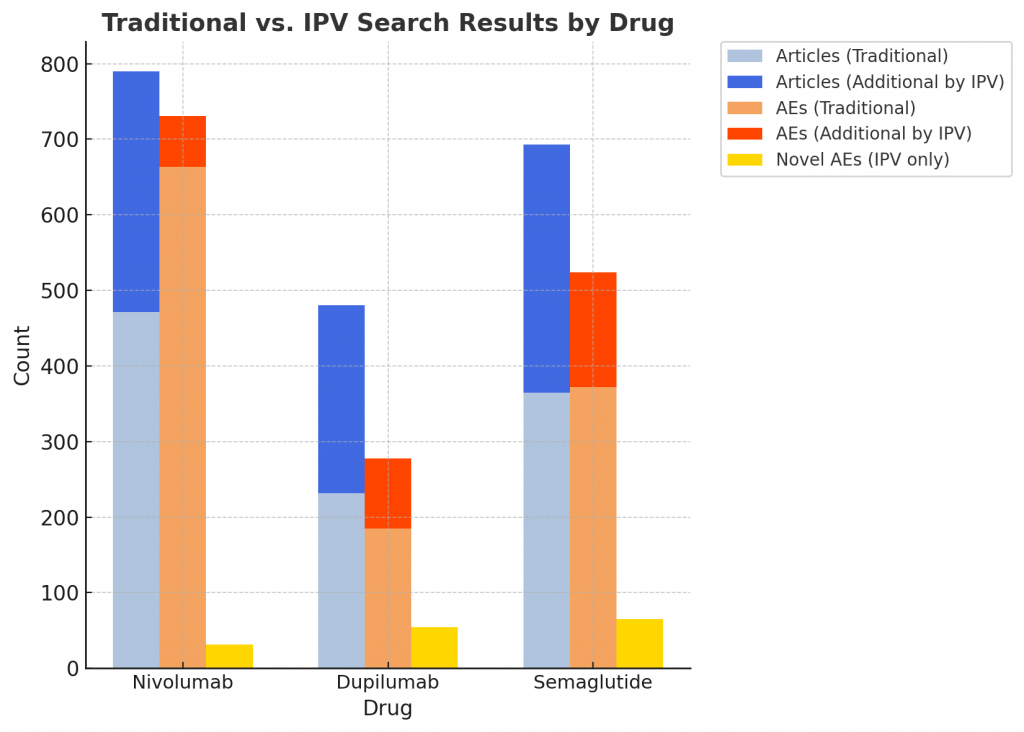
In a pilot study, our team compared Insilicom’s IPV platform with traditional Boolean keyword searches for detecting adverse events (AEs).
We focused on three well-known post-market drugs:
💊 Nivolumab, Dupilumab, and Semaglutide.
________________________________________
🔍 What we tested
• Both methods used the same drug names/synonyms and time window
• Traditional Boolean approach required “drug block + AE block” queries, due to the labor cost to manually read all the articles if AE block is not used.
• IPV searched only drug names and found nearly 2× more relevant publications. This allows more AEs to be detected.
Our AI method was used to extract AEs from all the articles found by both methods. No manual reviews were involved. From a benchmark dataset, we should that our AI method has a 98% recall when detecting articles with AEs.
________________________________________
📈 The results
30% more AEs detected — IPV uncovered critical literature missed by traditional Boolean searches
Greater efficiency — reduced manual review, faster turnaround, reproducible outcomes
________________________________________
🏛 Why it matters
We presented these findings at an FDA meeting, highlighting how AI can strengthen pharmacovigilance workflows and improve drug safety monitoring.
________________________________________
🚀 Want to see it in action?
We invite pharmacovigilance and drug safety teams to try IPV or run a pilot study — and experience how AI can transform your literature surveillance.
#Pharmacovigilance #DrugSafety #ArtificialIntelligence #LiteratureSurveillance #Nivolumab #Dupilumab #Semaglutide