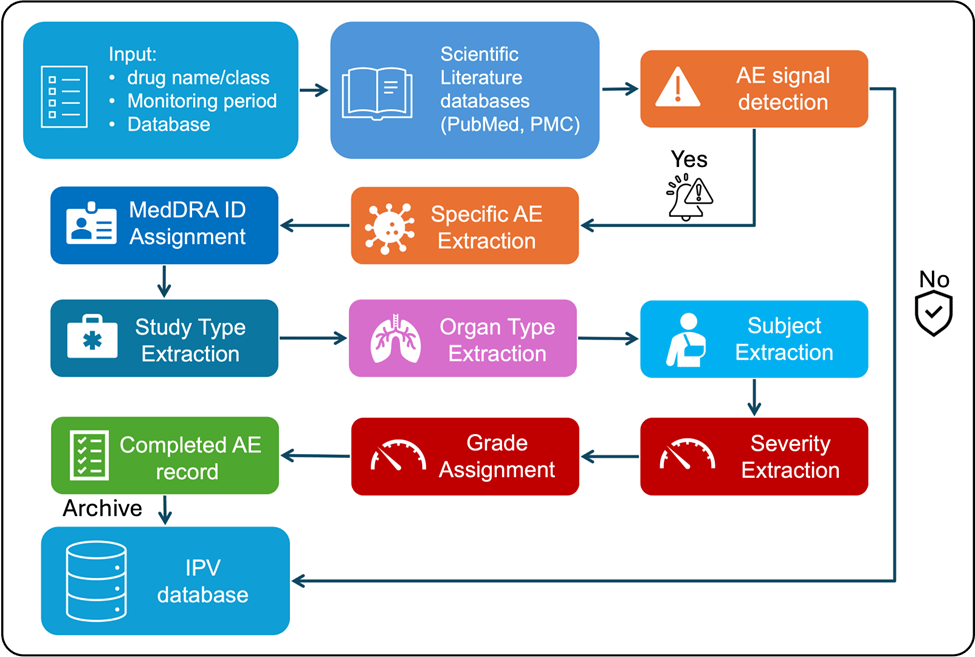
Many pharmacovigilance (PV) professionals still think AI is limited to search or summarization. But in reality, AI can support every step of the AE literature monitoring workflow.
Here’s how it works in our IPV system:
1️⃣ Document Retrieval
Given a drug or drug class, AI helps normalize entities — recognizing synonyms or “is-a” relationships (e.g., PD-1 inhibitors → nivolumab).
This ensures comprehensive and accurate retrieval.
2️⃣ Detecting AE-Relevant Papers
AI models can identify whether an article truly discusses AEs.
A high-recall model is essential here — we don’t want to miss relevant literature.
3️⃣ Extracting Specific AEs
Instead of manual reading, AI can directly extract adverse events mentioned as caused by the drug — saving huge review time.
4️⃣ Mapping to MedDRA IDs
For AEs with no exact term match, AI finds the closest MedDRA concept automatically.
This is especially useful for novel or nuanced AE expressions.
5️⃣ Extracting Related Context
AI can also capture study type, organ system, subject population, and AE severity — enriching downstream analysis.
6️⃣ Auto-Generating Draft Reports
Finally, AI can summarize all findings, insert citations in the right places, and generate a structured report for PV scientists to review and approve.
💡One surprising observation:
Many PV scientists still don’t realize AI can extract previously unknown AEs from literature.
This gap between AI capabilities and PV awareness is exactly what we aim to bridge.
We’ll continue sharing practical examples to help the community understand what’s possible — and how AI can transform PV workflows.
👉 What part of this process do you think AI could improve the most?
#Pharmacovigilance #ArtificialIntelligence #DrugSafety #AIAutomation #MedDRA #AdverseEventsMonitoring